Study Advertising
Leverage personalized, cost-effective study advertising services to quickly gather qualified candidates.

Field-tested clinical trial marketing and media outreach strategies that speed your enrollment.
WCG’s Study Advertising solution gives you the media resources and clinical trial marketing expertise necessary to keep participant recruitment on schedule. Across therapeutic areas, we provide an unmatched experience in devising high-impact media and community marketing campaigns.
How WCG Study Advertising Accelerates Enrollment
Smart Program Design
With the need for flexibility, we maintain a centrally managed, locally deployed solution, always with the participants’ journey in mind.
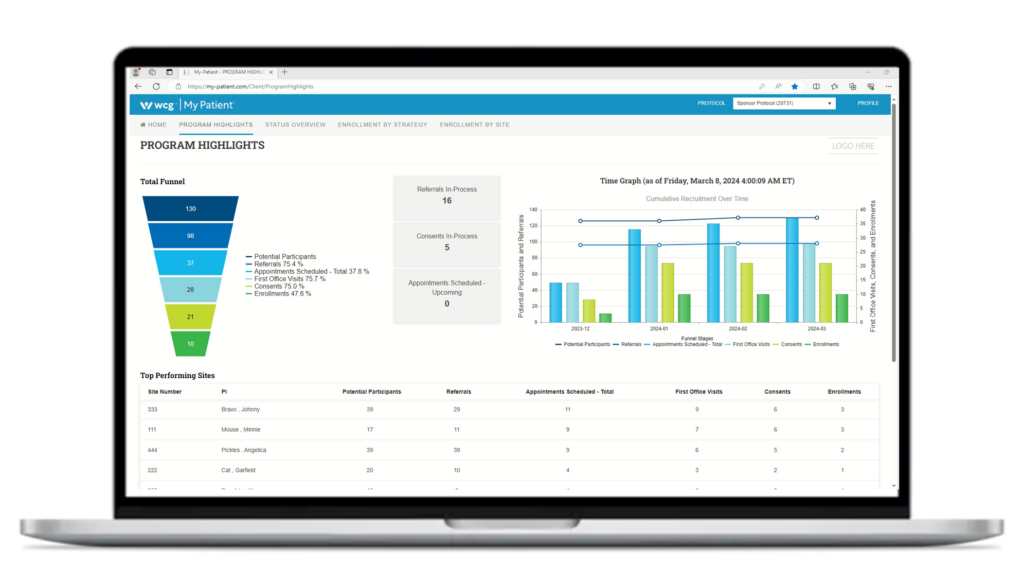
Actionable Metrics
Looking beyond top-of-the-funnel metrics like impressions and click-through rate, our focus falls on high-impact study metrics such as referrals, office visits, consents, and enrollments. Equally important to the enrollment numbers are the reasons for disqualification to allow for pivots and changes in the overall process.
Rapid Deployment
We understand that clinical study time is precious. WCG stands ready to respond rapidly to your circumstances and apply the most targeted and cost-effective media outreach strategies available to immediately generate a flow of qualified participant candidates.
Always Transparent. Always Accountable.
Using My Patient®, WCG’s web-based CRC workflow and participant management solution, you’re able to monitor every stage of the participant journey—in real-time at the most granular level possible—so you always feel in control.
Built-for-purpose, high-impact media and clinical trial advertising solutions for your studies
Complete the form to schedule a consultation with WCG.


